Abstract
BACKGROUND: Acute myeloid leukemia (AML) arises from diverse genetic and epigenetic abnormalities, resulting in heterogeneous response to treatment and variable patient survival. Within the general AML population, ~30% have mutations in the gene FMS-like tyrosine kinase 3 (FLT3), often as secondary events linked to leukemic transformation. These mutations either develop or become undetectable during the disease course, and typically the mutant-to-wild-type FLT3 allelic ratio also changes. The dynamics of this FLT3 clonal evolution and the clinical consequences are not fully understood, especially in the era of newer targeted therapies.
OBJECTIVE: Broadly, the aim of the study is to characterize the natural history of FLT3 mutations in AML from diagnosis through treatment for a 36-month period. The primary objective is to determine the proportion of relapsed/refractory AML patients with FLT3 clonal evolution (gain/loss of FLT3 mutations) from diagnosis. Here, we present results from the first interim analysis conducted 12 months after study initiation.
METHODS: This is a non-interventional, prospective study of adults (≥18 years) with AML diagnosed ≤30 days pre-enrollment at sites in Belgium, France, Germany, Israel, Italy, Spain, the UK and the USA. Assuming that 50% of patients will experience FLT3 clonal evolution, a 5% margin of error, and a 60% FLT3 retesting rate, 650 patients would need to be enrolled. Patients with relapsed/refractory AML at trial entry, acute promyelocytic leukemia, or central nervous system leukemia were excluded. Patients receive treatment according to local standard of care, at the treating physician's discretion. Routine FLT3 genetic testing is performed at diagnosis and at relapse/refractory disease. All AML treatments are recorded. Secondary objectives: frequency, allelic ratio, length and location of FLT3-internal tandem duplication (ITD) mutations, and frequency of other FLT3 mutations, at initial AML diagnosis and at relapse/refractory disease; frequency of FLT3 clonal evolution by mutation type and AML treatment(s) received; patient survival by FLT3 mutation status at diagnosis, FLT3 clonal evolution, and AML treatment(s) received; frequency of composite complete remission (includes complete remission [CR], CR with incomplete hematological recovery [CRi], and CR with incomplete platelet recovery [CRp]), by FLT3 mutation status at diagnosis, FLT3 clonal evolution, and AML treatment(s) received; and frequency and characteristics of other AML-associated genetic mutations at diagnosis and relapse/refractory event. Informed consent was obtained from all patients.
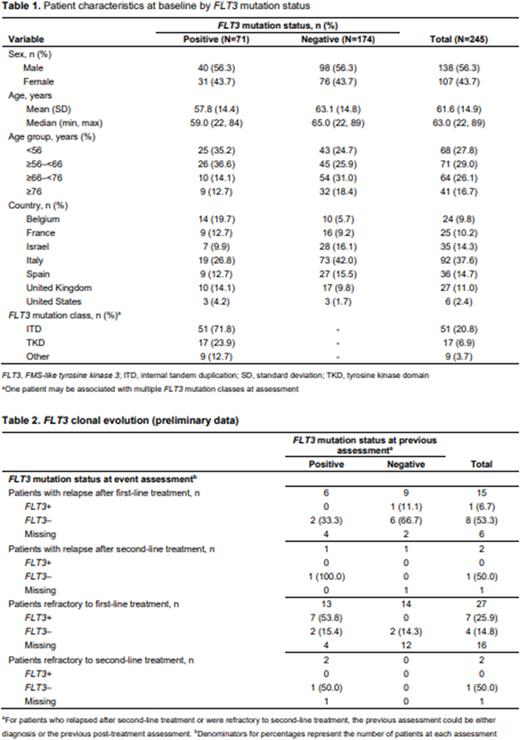
RESULTS: At data cutoff (September 30, 2021), 272 patients were enrolled; 245 had ≥1 post-enrollment data point and were included in the full analysis set (Table 1). More patients were male (56.3%, 138/245), and the median (min, max) age was 63.0 (22, 89) years. The patient geographical distribution is given in Table 1. At baseline, 29.0% (71/245) of patients tested positive for FLT3 mutations (FLT3+), and of these, 71.8% (51/71) had an FLT3-ITD mutation (Table 1). A higher proportion of patients <56 or ≥56-<66 years tested FLT3+ vs FLT3−, and vice versa for patients ≥66-<76 or ≥76 years.
At the time of the interim analysis, 15 (6.1%; 6 FLT3+, 9 FLT3− at diagnosis) patients had relapsed after first-line treatment and 27 (11.0%; 13 FLT3+, 14 FLT3− at diagnosis) patients were refractory after first-line treatment. Of the primary refractory patients, two (both FLT3+ at diagnosis) were refractory after second-line treatment and two (1 FLT3+, 1 FLT3− at diagnosis) experienced a relapse after second-line treatment. Preliminary findings regarding FLT3 clonal evolution by relapse/refractory event are shown in Table 2. The number of patients and relapse/refractory events in this first interim analysis is very limited; also, FLT3 test results are not yet available or testing was not done for 40% of the patients who relapsed and 59% of those who were refractory after first-line treatment. Therefore, the full extent of FLT3 clonal evolution remains uncertain and will need to be further analyzed in later data cuts.
CONCLUSIONS: This ongoing study, which has now recruited all required 650 patients, will help better understand the frequency of FLT3 mutations and the extent of FLT3 clonal evolution throughout the AML disease course, and the potential impact of these mutations on patient survival.
Disclosures
Papayannidis:Astellas: Membership on an entity's Board of Directors or advisory committees. Canaani:Astellas: Other: Consulting fees; AbbVie: Other: Consulting fees. De Becker:Pfizer: Other: Received support for congress attendance; BMS: Other: Received support for congress attendance; Takeda: Other: Received support for congress attendance; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Tandra:Alexion Pharmaceuticals: Honoraria; ADC Therapeutics: Honoraria. Elsouda:Astellas: Current Employment. Houweling:Astellas: Current Employment. Upadhyay:Astellas: Current Employment. Wouters:Astellas: Current Employment. Vyas:Celgene: Honoraria, Research Funding; JAZZ: Honoraria; Abbvie: Honoraria; Astellas: Honoraria; Daiichi Sankyo: Honoraria; Pfizer: Honoraria; Bristol Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal